Bioanalytical Testing Services Market was valued at US$ 4.63 Bn. in 2023, and it is expected to reach US$ 8.02 Bn by 2030, exhibiting a CAGR of 8.18 % during the forecast period
Bioanalytical Testing Services Market Report Overview:
The report comprehensively encompasses the analysis of insights concerning the Bioanalytical Testing Services Market , including its dynamic patterns, industry landscape, and all significant aspects of the market. An in-depth examination of key players is also presented within the Bioanalytical Testing Services Market report.
Request a sample report: https://www.maximizemarketresearch.com/request-sample/108536/
Bioanalytical Testing Services Market Scope and Research Methodology
The aim of this report is to assess and predict the size of the Bioanalytical Testing Services Market . It offers strategic profiles of significant market participants to provide an accurate depiction of the competitive landscape within the global Bioanalytical Testing Services Market . This includes a comprehensive analysis of recent developments such as new product launches, acquisitions, mergers, joint ventures, brand activities, and major players in the Bioanalytical Testing Services Market industry. The report presents insights into industry trends, dynamics, and potentials, assisting professionals in staying informed about the latest trends and sector performance. This insight aids in predicting growth and decline in Bioanalytical Testing Services Market share over the forecast period.
In-depth understanding of the Bioanalytical Testing Services Market industry was achieved through a combination of primary and secondary research methods. Various methodologies, including PESTLE, PORTER, and SWOT analysis, were employed to ensure accurate findings. SWOT analysis was employed to outline strengths, weaknesses, opportunities, and challenges for key players within the Bioanalytical Testing Services Market industry. Additionally, the use of PORTER and PESTLE analysis allowed for an understanding of the microeconomic and macroeconomic factors influencing the Bioanalytical Testing Services Market industry.
Bioanalytical Testing Services Market Segmentation:
by Application
Oncology
Neurology
Infectious Diseases
Gastroenterology
Cardiology
Other Applications
by End-User
Pharmaceutical and Biopharmaceutical Companies
Contract Development and Manufacturing Organizations
Contract Research Organizations
Get your sample report now : https://www.maximizemarketresearch.com/request-sample/108536/
Bioanalytical Testing Services Market Key Players:
1. ICON plc
2. Covance Inc.
3. LabCorp
4. Charles River Laboratories International, Inc.
5. inVentiv Health
6. SGS SA
7. Toxikon, Inc.
8. Intertek group
9. Pace Analytical Services, LLC
10. Medpace
11. WuXi AppTec
12. Eurofins Scientific
13. IQVIA
14. Laboratory Corporation of America Holdings
15. Intertek Group
16. Syneos Health
17. Frontage Labs
18. PPD
19. PAREXEL International Corporation
20. Almac Group
Bioanalytical Testing Services Market Regional Analysis:
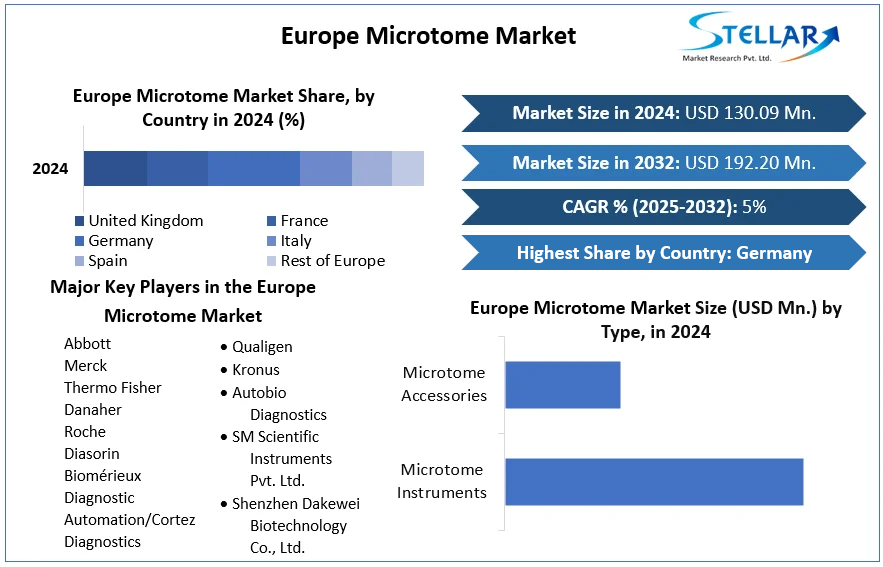
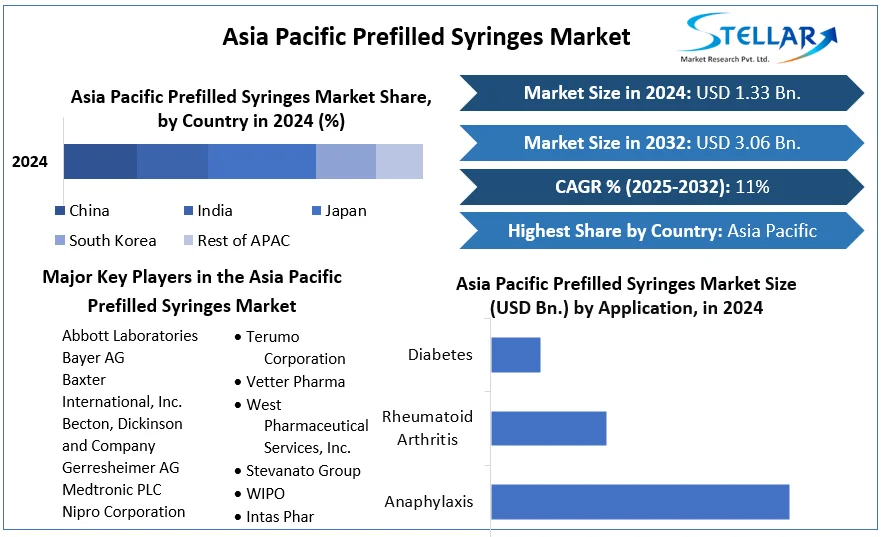
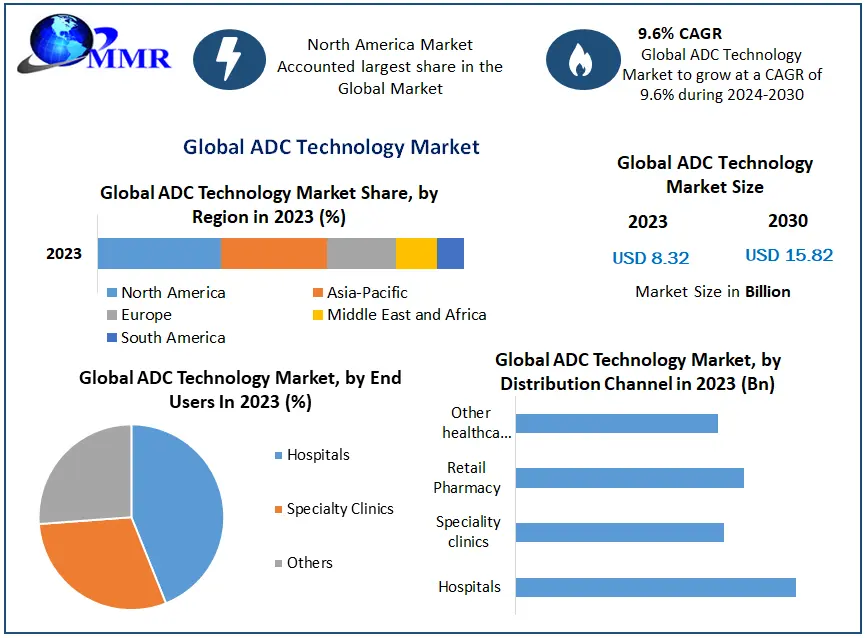
The report is segmented into several key countries, with market size, growth rate, import and export of Bioanalytical Testing Services Market in these countries, which covering North America, U.S., Canada, Mexico, Europe, UK, Germany, France, Spain, Italy, Rest of Europe, Asia Pacific, China, India, Japan, Australia, South Korea, ASEAN Countries, Rest of APAC, South America, Brazil, and Middle East and Africa.
Download your free sample : https://www.maximizemarketresearch.com/request-sample/108536/
Key Questions answered in the Bioanalytical Testing Services Market Report are:
Which segment grabbed the largest share in the Bioanalytical Testing Services Market ?
Which segment is expected to grow at a high rate during the forecast period?
How is the competitive scenario of the Bioanalytical Testing Services Market ?
Which are the key factors driving the Bioanalytical Testing Services Market growth?
Which are the factors restraining the Bioanalytical Testing Services Market growth?
Which region holds the maximum share in the Bioanalytical Testing Services Market ?
What will be the CAGR of the Bioanalytical Testing Services Market during the forecast period?
Which are the prominent players in the Bioanalytical Testing Services Market ?
Key Offerings:
A detailed Analysis of the Market Overview
Market Share, Size & Forecast by Revenue | 2024−2030
Market Dynamics – Growth Drivers, Restraints, Investment Opportunities, and Key Trends
Market Segmentation – A detailed analysis by Route of administration, Application, Facility of use and Region and Region
Competitive Landscape – Top Key Vendors and Other Prominent Vendors
About Maximize Market Research:
Maximize Market Research is a multifaceted market research and consulting company with professionals from several industries. Some of the industries we cover include medical devices, pharmaceutical manufacturers, science and engineering, electronic components, industrial equipment, technology and communication, cars and automobiles, chemical products and substances, general merchandise, beverages, personal care, and automated systems. To mention a few, we provide market-verified industry estimations, technical trend analysis, crucial market research, strategic advice, competition analysis, production and demand analysis, and client impact studies.
Contact Maximize Market Research:
3rd Floor, Navale IT Park, Phase 2
Pune Banglore Highway, Narhe,
Pune, Maharashtra 411041, India
[email protected]
+91 96071 95908, +91 9607365656
Bioanalytical Testing Services Market Report Overview:
The report comprehensively encompasses the analysis of insights concerning the Bioanalytical Testing Services Market , including its dynamic patterns, industry landscape, and all significant aspects of the market. An in-depth examination of key players is also presented within the Bioanalytical Testing Services Market report.
Request a sample report: https://www.maximizemarketresearch.com/request-sample/108536/
Bioanalytical Testing Services Market Scope and Research Methodology
The aim of this report is to assess and predict the size of the Bioanalytical Testing Services Market . It offers strategic profiles of significant market participants to provide an accurate depiction of the competitive landscape within the global Bioanalytical Testing Services Market . This includes a comprehensive analysis of recent developments such as new product launches, acquisitions, mergers, joint ventures, brand activities, and major players in the Bioanalytical Testing Services Market industry. The report presents insights into industry trends, dynamics, and potentials, assisting professionals in staying informed about the latest trends and sector performance. This insight aids in predicting growth and decline in Bioanalytical Testing Services Market share over the forecast period.
In-depth understanding of the Bioanalytical Testing Services Market industry was achieved through a combination of primary and secondary research methods. Various methodologies, including PESTLE, PORTER, and SWOT analysis, were employed to ensure accurate findings. SWOT analysis was employed to outline strengths, weaknesses, opportunities, and challenges for key players within the Bioanalytical Testing Services Market industry. Additionally, the use of PORTER and PESTLE analysis allowed for an understanding of the microeconomic and macroeconomic factors influencing the Bioanalytical Testing Services Market industry.
Bioanalytical Testing Services Market Segmentation:
by Application
Oncology
Neurology
Infectious Diseases
Gastroenterology
Cardiology
Other Applications
by End-User
Pharmaceutical and Biopharmaceutical Companies
Contract Development and Manufacturing Organizations
Contract Research Organizations
Get your sample report now : https://www.maximizemarketresearch.com/request-sample/108536/
Bioanalytical Testing Services Market Key Players:
1. ICON plc
2. Covance Inc.
3. LabCorp
4. Charles River Laboratories International, Inc.
5. inVentiv Health
6. SGS SA
7. Toxikon, Inc.
8. Intertek group
9. Pace Analytical Services, LLC
10. Medpace
11. WuXi AppTec
12. Eurofins Scientific
13. IQVIA
14. Laboratory Corporation of America Holdings
15. Intertek Group
16. Syneos Health
17. Frontage Labs
18. PPD
19. PAREXEL International Corporation
20. Almac Group
Bioanalytical Testing Services Market Regional Analysis:
The report is segmented into several key countries, with market size, growth rate, import and export of Bioanalytical Testing Services Market in these countries, which covering North America, U.S., Canada, Mexico, Europe, UK, Germany, France, Spain, Italy, Rest of Europe, Asia Pacific, China, India, Japan, Australia, South Korea, ASEAN Countries, Rest of APAC, South America, Brazil, and Middle East and Africa.
Download your free sample : https://www.maximizemarketresearch.com/request-sample/108536/
Key Questions answered in the Bioanalytical Testing Services Market Report are:
Which segment grabbed the largest share in the Bioanalytical Testing Services Market ?
Which segment is expected to grow at a high rate during the forecast period?
How is the competitive scenario of the Bioanalytical Testing Services Market ?
Which are the key factors driving the Bioanalytical Testing Services Market growth?
Which are the factors restraining the Bioanalytical Testing Services Market growth?
Which region holds the maximum share in the Bioanalytical Testing Services Market ?
What will be the CAGR of the Bioanalytical Testing Services Market during the forecast period?
Which are the prominent players in the Bioanalytical Testing Services Market ?
Key Offerings:
A detailed Analysis of the Market Overview
Market Share, Size & Forecast by Revenue | 2024−2030
Market Dynamics – Growth Drivers, Restraints, Investment Opportunities, and Key Trends
Market Segmentation – A detailed analysis by Route of administration, Application, Facility of use and Region and Region
Competitive Landscape – Top Key Vendors and Other Prominent Vendors
About Maximize Market Research:
Maximize Market Research is a multifaceted market research and consulting company with professionals from several industries. Some of the industries we cover include medical devices, pharmaceutical manufacturers, science and engineering, electronic components, industrial equipment, technology and communication, cars and automobiles, chemical products and substances, general merchandise, beverages, personal care, and automated systems. To mention a few, we provide market-verified industry estimations, technical trend analysis, crucial market research, strategic advice, competition analysis, production and demand analysis, and client impact studies.
Contact Maximize Market Research:
3rd Floor, Navale IT Park, Phase 2
Pune Banglore Highway, Narhe,
Pune, Maharashtra 411041, India
[email protected]
+91 96071 95908, +91 9607365656
Bioanalytical Testing Services Market was valued at US$ 4.63 Bn. in 2023, and it is expected to reach US$ 8.02 Bn by 2030, exhibiting a CAGR of 8.18 % during the forecast period
Bioanalytical Testing Services Market Report Overview:
The report comprehensively encompasses the analysis of insights concerning the Bioanalytical Testing Services Market , including its dynamic patterns, industry landscape, and all significant aspects of the market. An in-depth examination of key players is also presented within the Bioanalytical Testing Services Market report.
Request a sample report: https://www.maximizemarketresearch.com/request-sample/108536/
Bioanalytical Testing Services Market Scope and Research Methodology
The aim of this report is to assess and predict the size of the Bioanalytical Testing Services Market . It offers strategic profiles of significant market participants to provide an accurate depiction of the competitive landscape within the global Bioanalytical Testing Services Market . This includes a comprehensive analysis of recent developments such as new product launches, acquisitions, mergers, joint ventures, brand activities, and major players in the Bioanalytical Testing Services Market industry. The report presents insights into industry trends, dynamics, and potentials, assisting professionals in staying informed about the latest trends and sector performance. This insight aids in predicting growth and decline in Bioanalytical Testing Services Market share over the forecast period.
In-depth understanding of the Bioanalytical Testing Services Market industry was achieved through a combination of primary and secondary research methods. Various methodologies, including PESTLE, PORTER, and SWOT analysis, were employed to ensure accurate findings. SWOT analysis was employed to outline strengths, weaknesses, opportunities, and challenges for key players within the Bioanalytical Testing Services Market industry. Additionally, the use of PORTER and PESTLE analysis allowed for an understanding of the microeconomic and macroeconomic factors influencing the Bioanalytical Testing Services Market industry.
Bioanalytical Testing Services Market Segmentation:
by Application
Oncology
Neurology
Infectious Diseases
Gastroenterology
Cardiology
Other Applications
by End-User
Pharmaceutical and Biopharmaceutical Companies
Contract Development and Manufacturing Organizations
Contract Research Organizations
Get your sample report now : https://www.maximizemarketresearch.com/request-sample/108536/
Bioanalytical Testing Services Market Key Players:
1. ICON plc
2. Covance Inc.
3. LabCorp
4. Charles River Laboratories International, Inc.
5. inVentiv Health
6. SGS SA
7. Toxikon, Inc.
8. Intertek group
9. Pace Analytical Services, LLC
10. Medpace
11. WuXi AppTec
12. Eurofins Scientific
13. IQVIA
14. Laboratory Corporation of America Holdings
15. Intertek Group
16. Syneos Health
17. Frontage Labs
18. PPD
19. PAREXEL International Corporation
20. Almac Group
Bioanalytical Testing Services Market Regional Analysis:
The report is segmented into several key countries, with market size, growth rate, import and export of Bioanalytical Testing Services Market in these countries, which covering North America, U.S., Canada, Mexico, Europe, UK, Germany, France, Spain, Italy, Rest of Europe, Asia Pacific, China, India, Japan, Australia, South Korea, ASEAN Countries, Rest of APAC, South America, Brazil, and Middle East and Africa.
Download your free sample : https://www.maximizemarketresearch.com/request-sample/108536/
Key Questions answered in the Bioanalytical Testing Services Market Report are:
Which segment grabbed the largest share in the Bioanalytical Testing Services Market ?
Which segment is expected to grow at a high rate during the forecast period?
How is the competitive scenario of the Bioanalytical Testing Services Market ?
Which are the key factors driving the Bioanalytical Testing Services Market growth?
Which are the factors restraining the Bioanalytical Testing Services Market growth?
Which region holds the maximum share in the Bioanalytical Testing Services Market ?
What will be the CAGR of the Bioanalytical Testing Services Market during the forecast period?
Which are the prominent players in the Bioanalytical Testing Services Market ?
Key Offerings:
A detailed Analysis of the Market Overview
Market Share, Size & Forecast by Revenue | 2024−2030
Market Dynamics – Growth Drivers, Restraints, Investment Opportunities, and Key Trends
Market Segmentation – A detailed analysis by Route of administration, Application, Facility of use and Region and Region
Competitive Landscape – Top Key Vendors and Other Prominent Vendors
About Maximize Market Research:
Maximize Market Research is a multifaceted market research and consulting company with professionals from several industries. Some of the industries we cover include medical devices, pharmaceutical manufacturers, science and engineering, electronic components, industrial equipment, technology and communication, cars and automobiles, chemical products and substances, general merchandise, beverages, personal care, and automated systems. To mention a few, we provide market-verified industry estimations, technical trend analysis, crucial market research, strategic advice, competition analysis, production and demand analysis, and client impact studies.
Contact Maximize Market Research:
3rd Floor, Navale IT Park, Phase 2
Pune Banglore Highway, Narhe,
Pune, Maharashtra 411041, India
[email protected]
+91 96071 95908, +91 9607365656
0 Reacties
0 aandelen
4401 Views