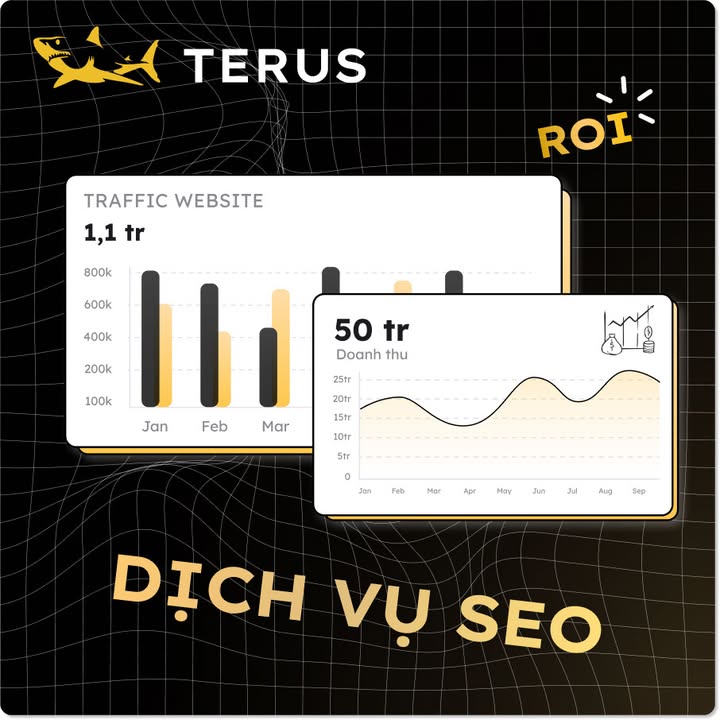
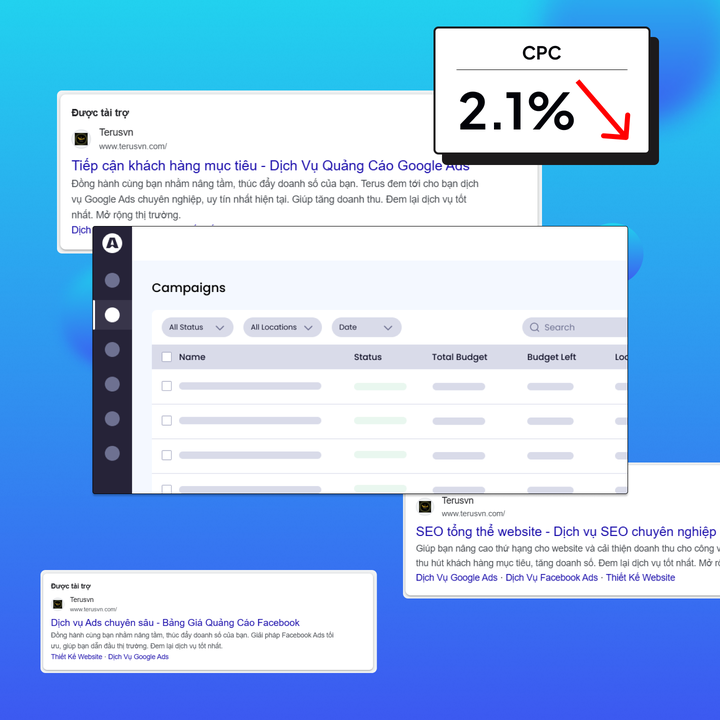
Đường đi của bão số 5 năm 2025: dự báo từ 13h ngày 23/8 đến 13h ngày 26/8/2025
https://tnnet.vn/duong-di-cua-bao-so-5-nam-2025-du-bao-tu-13h-ngay-23-8-den-13h-ngay-26-8-2025
https://tnnet.vn/duong-di-cua-bao-so-5-nam-2025-du-bao-tu-13h-ngay-23-8-den-13h-ngay-26-8-2025
Đường đi của bão số 5 năm 2025: dự báo từ 13h ngày 23/8 đến 13h ngày 26/8/2025
https://tnnet.vn/duong-di-cua-bao-so-5-nam-2025-du-bao-tu-13h-ngay-23-8-den-13h-ngay-26-8-2025
0 Comments
0 Shares
5 Views